-
Asked by to Aimee, Chris, Dave, Greig, Laurence on 24 Jun 2014. This question was also asked by .0
-
Greig Cowan answered on 24 Jun 2014:
Hi Swagy. This is known as the Mpemba effect. Although it has been observed by a number of people and can be repeated, it is not clear at all what causes it! It could be due to evaporation of water from the surface of the hot water, or due to supercooling of the cold water relative to the hot water, or due to the particular mixture of salts that are dissolved in the water. Maybe you could try doing some experiments yourself 🙂
-
-
Aimee Hopper answered on 24 Jun 2014:
No-one really knows why exactly, but it is believed that the hot water can do a few things that the cold cant. for example
1) the hot water may melt the ice build-up on top of the cooling elements, and so has a better contact with the cooling element and so cools faster.
2) convection currents in the hot water enable it to cool down faster.So even though the water starts at a hotter temperature, it cools down faster.
Obviously though if the hot water started at 99 degrees C and cold at 0.1 degrees C, the cold water will freeze first
-
Laurence Perreault Levasseur answered on 24 Jun 2014:
As the other said, this is called the Mpemba effect. It’s called after the student from Tanzania who first published a paper about it. He realized that hot ice cream mix freezes faster than hot ice cream mix (that’s a pretty cool experiment, if you want my opinion!!). You can try it for yourself in the winter, but it has to be a cold day. Check out a home-made backyard version of the experiment:
Ok now, why does this happen?? It definitely seems counter intuitive! And it’s also been a huge puzzle for many years for lot of chemists and physicists! There has been a lot of explanations hypothesized, like:
– faster evaporation of hot water, which takes lots of heat away form the water and also reduces the volume of water left to freeze;
– when cold water freezes it might form a layer of ice on it, which acts like an insulator and prevents it from cooling further;
– different concentrations of CO2 and other gasses dissolved in the water. when the water is heated, all those gases get expelled form the water, while cold water still has lots of gases dissolved in it.
But really, until recently, no one had really understood why this happens. Turns out, apparently it’s none of these things!!Last year, there is a group of researchers from Singapore that claimed to have finally found the explanation:
In liquid water there is 2 types of bonds between atoms.1) the covalent bounds that link H with O, that’s why water is H2O. Basically each H ends up sharing it’s electron with the O, to fill the valence level (i don’t know if you’ve seen that in chemistry, if not, it’s not important). That bond is VERY strong.
2) when H share an electron with O, the electron is kind of between the 2 atoms, so that the H atom looks more positive from the outside (because of the + charge of the proton, since the electron with its – charge is further away). On the other hand, the O atom looks more negative, because on top of it’s usual electron, it has an extra 2 that it’s sharing. So it looks more negative. This means that an H (overall more +) from ONE H2O molecule can be attracted to the O (overall more -) from ANOTHER water molecule. This bond is called an H bond. Usually it’s very weak, and exciting the molecules a bit will destroy it very easily.
Here is an image of the 2 types of bonds. Red is O, grey is H, the dotted likes are the H-bonds, and the solid lines are the covalent bonds.
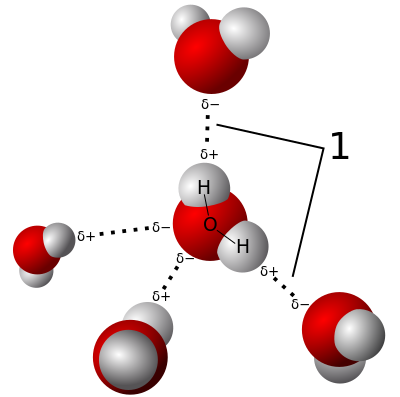
Ok so the trick here is about an interplay between those 2 types of bonds. In cold water, the water molecules are not very excited, so they will tend to hang out closer to each other. That means, with time, there are more chances they form H-bonds. BUT, when they form those H-bonds, the 2 water molecules get kind of repelled (the rest of the atoms that are not part of the H-bond don’t want to be close to the other water molecule), so the covalent bonds get stretched. This stretching stores energy, and in order to freeze, the water molecules have to give off all of that energy, which takes time (and it’s hard to get rid of that extra energy!!).
Hot water, however, have much more excited molecules. They fly all over the place, and are much further away than the cold water molecules. That means they form much less H-bonds. So the covalent bonds of the H2O molecules aren’t stretched like in cold water, and they store much less energy. So hot water has a much easier time cooling down because it doesn’t have all that extra energy to get rid of.
The researchers who did this research claim that this energy storage explains the difference in freezing times between hot and cold water 😀
You can look for more info here:
http://www.iflscience.com/chemistry/hot-water-freezes-faster-cold-and-now-we-know-why

Comments